Potency pains and how internal HPLC analysis can help

In California alone, dozens of analytical laboratories can be found specializing in hemp and cannabis testing offering a full suite of analysis including potency, residual solvents, terpenes, moisture, and more. Most, if not all these laboratories have achieved ISO 17025 certifications, an internationally recognized standard for competency in testing and calibration laboratories. Although practices for applying standard analytical techniques to measure different analytes have been developed into a standard certification, the application of these techniques to hemp and cannabis samples and the wide array of analytes contained within these process streams have not.
Searching for HPLC methods for quantification of cannabinoids yields numerous results from academic papers suggesting optimized methods to white papers published by nearly every HPLC equipment and supply manufacturer. With all these proposed methods, who is to say which is the best? Is there even a best method? It’s up to each analytical laboratory to choose the right method suitable for their equipment and throughput, balancing separation efficiency and acquisition time. There is a greater incentive placed on a faster runtime as mobile phases like methanol or acetonitrile are running through to waste every minute the instrument is in use, so sacrificing separation efficiency for shorter acquisition times has a real cost benefit.
There are many factors involved in developing an HPLC method, from the instrument used, to column type, mobile phases, gradient parameters and many more. A laboratory with a large budget may be able to utilize UHPLC pressures to achieve higher separation efficiency while someone relying on older equipment may need to rely on slow isocratic methods. Hemp and cannabis testing also requires that these key analytes be measured in drastically different process streams. From an analytical chemistry perspective, sample preparation of flower for cannabinoid analysis requires significantly different procedures than preparing a crude concentrate for analysis. Not only do the key analyte concentrations differ widely, so do the impurity profiles. Not only that, but concentrates created by different manufacturing processes can vary widely in their impurities, causing potential for coeluting peaks in a method from one source of crude oil to another. All this together shows the near impracticality of developing an optimal method to account for all these potential variables.
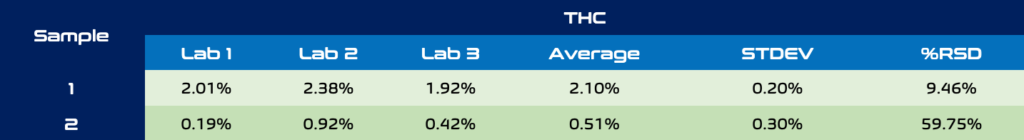
Any business in the hemp and cannabis industry can speak to the variability in external lab results. Usually the go-to test is to send out replicates of a sample to multiple analytical labs to compare against each other. Hopefully at least two labs come back with comparable results and if not, whose to trust? Table 1 and 2 below show CBD and THC concentrations of two different CBD crude oil samples sent to 3 ISO 17025 certified testing labs. The standard deviation for CBD for each sample was +/- 5%, although lab 2 looks to be a clear outlier for CBD with labs 1 and 3 being within 2-4% of each other for both samples. THC analysis in hemp derived extracts can be especially difficult to quantify by external labs, especially at levels < 0.3%. These sensitive results can be the difference between compliant and non-compliant final products. Sample 3 is a fitting example of this as the THC concentrations show significantly differing results. Only one is below 0.3% compliance, while at the other end THC was measured as 3x the compliance limit! The costs of doing these replicate tests across laboratories and even providing blind replicates to a single testing lab can add up quickly and may not even provide the level of confidence needed to get products to market.
Internal HPLC capabilities can provide that high level of confidence at each stage in the process through traceability in reference standards and ease of preparing and analyzing replicates. A capable chemist can prepare and analyze dozens of samples in an 8-hour shift. Going further, the chemist can develop methods better suited to a particular process stream, ensuring analytes of interest, such as CBD and THC, do not have coelution issues with process specific impurities. The figure below is an example chromatogram showing the d9-THC peak in a crude concentrate sample at a concentration below 1000 ppm. Multiple peaks elute closely and if separation is not adequate, can easily be misidentified as d9-THC and inflate the measured concentration.
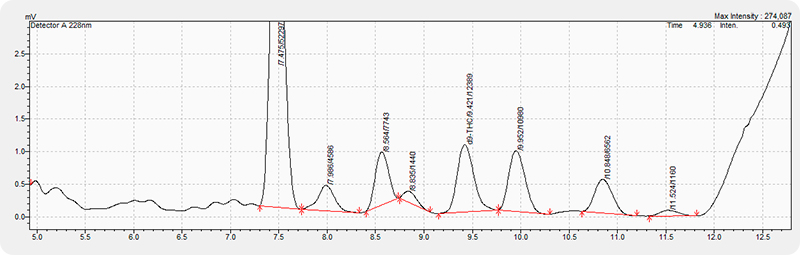
Relying on analytical laboratories alone to provide critical data leaves much to be desired in terms of validation and control of process parameters and final product quality. With cannabinoid profile usually being the critical process parameter, having the capabilities to tailor the analysis to a specific process flow is key. Understanding the compounds and peaks that are not reported on standard potency analysis can provide key insights to help optimize the process and ensure batch to batch consistency. Having to wait for external analysis and up to a week for data turn-around can result in lost time and even lost product. There will always be a place for external laboratory testing to provide confidence to customers and validation of internal testing, but without industry standard methods and protocols, it should not be relied upon as absolute truth.
Table 1. CBD analysis across 3 different ISO 17025 certified laboratories.
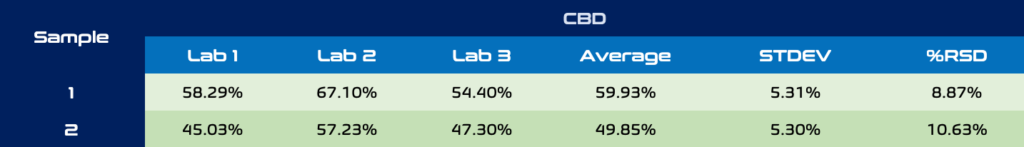
Table 2. THC analysis across 3 different ISO 17025 certified laboratories.
Contact us for more information
Contact our sales team for more information on custom CBD extraction systems, state-of-the-art THC remediation systems or preview our catalog of systems and modules available through our website.
Related Topics
- Analysis
- Cannabis Industry
- CBC Extraction
- CBD Extraction
- cbd isolate
- CBG Extraction
- cGMP
- Chromatography
- CO2
- Cold Water Extraction
- Ethanol Extraction
- Extraction 101
- Extraction Equipment
- Extraction Machine
- Extraction System
- GMP
- GMP Certification
- Hemp Extraction
- HPLC
- Remediation
- Remediation Equipment
- Terpene Extraction
- terpenes
- THC
- THC Remediation
- All
- Extraction
- THC Remediation
- All
- Extraction
- THC Remediation
- Email: info @ entexs.com
- Toll Free: (888) 960-3689
- 3720 Trade Way, Cameron Park, CA 95682, USA
- ENTEXS is proud to be made in the USA
- © 2024 ENTEXS Corporation